Anti-Drug Immune Response (Immunogenicity)
Immunogenicity, in particular of protein-based or cell and gene therapy products, can impact therapy success and can even lead to life threatening safety problems. Therefore, it is of the utmost importance to monitor any putative generation of anti-drug immune responses such as e.g., anti-drug antibodies (ADA) and/or anti-drug T cells (ADT) and/or innate responses before and during clinical trials.
Anti-drug antibodies can impact on bioavailability and pharmacokinetics or can completely neutralize the activity of the drug depending on their binding site. ADA can even lead to anaphylactic shock as a result of their potential to activate complement, mast cells and basophils (depending on their isotype). Therefore, it is important to monitor ADA-responses in treated patients and determine their concentration or titer in addition to the isotype, if possible. For cell and gene therapy (CGT) products, assessing anti-drug T cells -responses is of particular interest due to interactions with the CGT product that can affect the survival and function of the CGT product, and subsequently impact its efficacy and safety. In addition, monitoring innate responses (e.g. cytokine release, complement activation) is particularly relevant for cell-and-gene therapy products or biologicals which target cells.
What we can add to your research
We have considerable experience in performing sponsor specific validated anti-drug antibody assays in preclinical as well as phase I up to phase III clinical studies in our laboratory. We offer advice and support to develop such anti-drug antibody assays according to EMA/FDA guidelines. Our assays can account for ADAs against the drug itself as well as for any modifications (e.g. PEGylation) or fusion parts to the drug or contaminations from factors or cell-lines, which have been used during production of the drug. The assays should be able to distinguish to which part the anti-drug antibodies would bind to. In addition, specificity of binding of antibodies will be assessed for screen-positive samples by antigen-competition in a very short turnaround time. We have different technologies to detect T-cell sensitization (anti-Drug Tcells) or innate responses to drugs, such as ELISpot and Flow cytometry, or Multi-Ligand assays.
Would you like to talk to us?
Test principles:
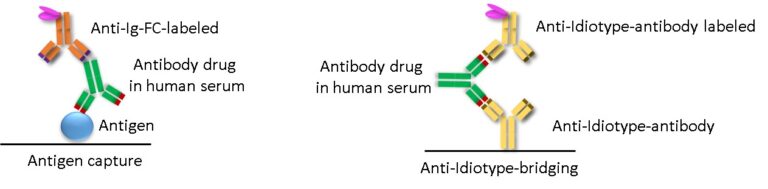
There are two main principles of ADA detection: ADA detection via binding to antigen (drug) coated onto plates (Fig.1) and bridging assays (Fig.2). Direct binding of the drug to the ELISA-plate as in Fig.1 allows isotype determination, which is not possible when using bridging assays.
Furthermore, we have a wealth of experience in the measurement of anti-drug T cell responses, e.g., against autologous/allogeneic cell products, viral vector-based gene therapy, and CAS proteins using different functional T-cell platforms (ELISpot, Multiparameter flow cytometry, scRNAseq), or innate responses such as cytokine release, activation of myeloid cells using different platforms (Multiligand assays, flow cytometry, scRNAseq).
Our Services
- Development of ADA assays or transfer of sponsor developed assays
- Validation of ADA assays
- Development of T-cell based anti-Drug Tcells assays
- Validation of T-cell based anti-Drug Tcells assays
- Validation of innate immunity tests
- Sample testing
- Screening assay with titer determination
- Confirmatory assays
Assay development and Validation Strategy:
Identify / Test positive control antibodies
Cut-off determination of the assay by testing negative samples
Precision assessment
- Intra-assay (within assay) Variances
- Inter-assay (between assays) Variances
Sensitivity of assay (based on positive control)
Robustness assessment
- Stability of samples (freeze/thaw cycles)
- Free drug tolerance
Equipment:

Mesoscale
(QuickPlex SQ120)

ELISA
(Molecular Devices 340pc)

EliSpot
Key Information
- 15 years of experience with ADA assays in clinical studies
- Development of customized ADA assays
- Full validation of ADA assays in accordance with EMA/FDA guide lines
- Analysing clinical samples with short turnaround time
- adhoc data generation via LIMS in short time
- 10 years of experience with ADT assays in clinical studies
- Development of customized ADT assays
- Validation of ADT assays
